
#HOMO VS LUMO SERIES#
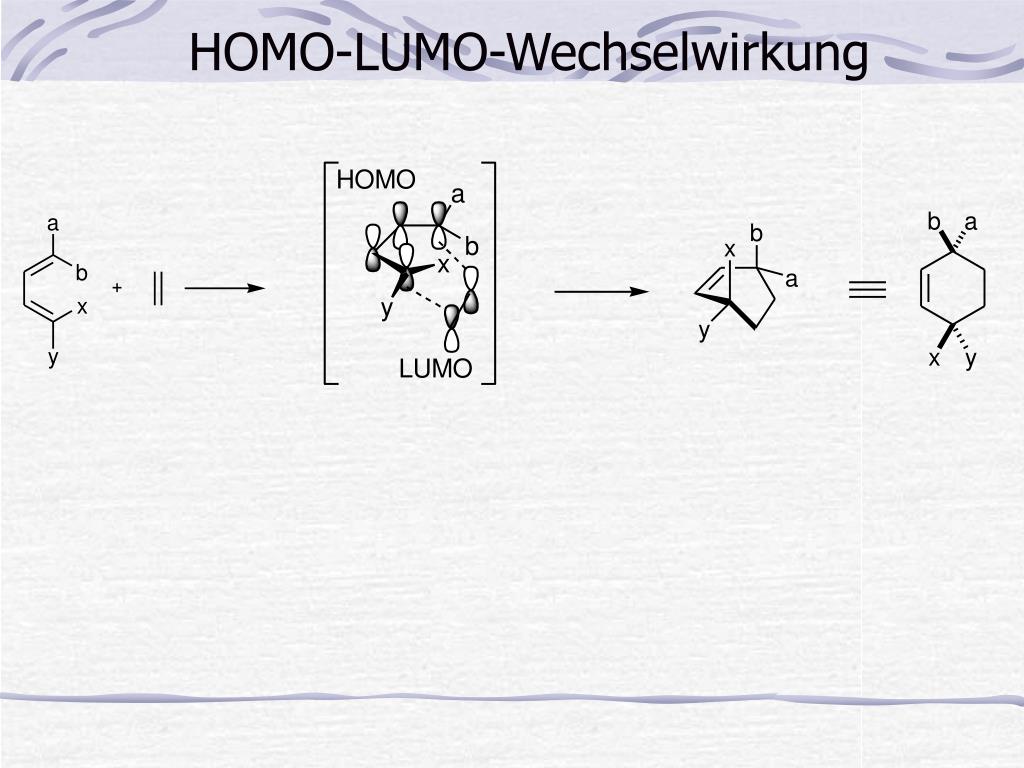
The LUMO and HOMO energies and their differences of this series of imine intermediates are shown in Table 1 (see Figure 4 for their structures).Īble 1. Next, we change the substitution position on the indole ring or replace the indole with various aromatic and hetero-aromatic systems, while holding the phenyl imino moiety unchanged. If there is no lobe distributed on HOMO or LUMO at the reaction sites, HOMO-1/HOMO-2 or LUMO+1/LUMO+2 with appropriate lobe distribution should be considered for the energy gap estimation. Reactivity can be gauged by the energy difference between the LUMO and HOMO orbitals, which is 8.1 eV for the structure in Figure 3. Interactions of these orbitals lead to the cyclization observed. Pictet-Spengler reaction (top) and LUMO orbital, HOMO orbital and energy of imine (bottom)Īs illustrated in Figure 3, there are HOMO lobe distributed between C2 and C3 of indole, and LUMO lobe on the imine carbon on the condensation intermediate. Pictet-Spengler reaction is the condensation of β-(hetero)aryl ethylamines with aldehydes or ketones under acidic conditions, followed by addition of the nucleophilic aromatic moiety of the molecule onto the resultant electrophile imine to provide the cyclization product (Figure 3, top).įigure 3. Analysis of a set of substrates for Pictet-Spengler reaction Next, we’ll make use of this LUMO-HOMO energy difference analysis to account for reactivity pattern observed with a set of substrates for Pictet-Spengler reaction. In other words, the nucleophilicity and electrophilicity of substrate/reagent can be assessed by their HOMO and LUMO energies, and whether the reaction will proceed or not could be judged by the difference in energy of the orbitals involved in the reactions. Why LUMO energy could be used to gauge chemical reactivity? Zhi-Xiang Yu’s group provided a theoretical understanding of the Mayr equation based on frontier molecular orbital theory (FMO) and the Eyring equation of the transition state theory, showing that the nucleophilicity of a molecule is related to the energy of this molecule’s highest occupied molecular orbital (HOMO), while the electrophilicity is related to the energy of the lowest unoccupied molecular orbital (LUMO) of the electrophile (Figure 2). A Frontier Molecular Orbital Theory approach to understanding the Mayr Equation Correlation between LUMO-HOMO energy difference and reactivityįigure 2. These calculated LUMO energy numbers correlate with chemical reactivity of the reagents: the lower the LUMO energy, the more reactive it is as halogenation reagent. TCCA has the lowest LUMO energy of -0.79 eV, DCH LUMO has an intermediate energy of 0.37 eV, and NCS has the highest energy of 1.09 eV. The LUMO lobes of these reagents are distributed around the chlorine atoms, not on the carbonyl carbons, consistent with the fact that they are halogenation reagents rather than acylation reagents. The calculated LUMO orbitals and energies of TCCA, DCH, and NCS are shown in Figure 1. LUMO orbitals and energies of TCCA, DCH and NCS How much stronger? Is there a way to quantify the differences?įigure 1. DCH and TCCA are stronger chlorination reagents than NCS. For example, halogenation of electron-rich aromatic substrates with N-chlorosuccinimide (NCS) usually works, while for electron-deficient ones, we’ll need to use dichlorohydantoin (DCH) or even trichloroisocyanuric acid (TCCA). In organic synthesis, we need to pair substrates and reagents of proper reactivity for the reactions to proceed smoothly.
Assessing Reactivity with LUMO and HOMO Energy Gap QM Magic Class | Chapter 25


 0 kommentar(er)
0 kommentar(er)
